Surfactants and What They Do
Why do we need to use cleaning solutions? Isn’t water good enough? What do the chemical solutions even do for us?
Obviously we all know that chemical solutions help us clean more effectively, but why is that?
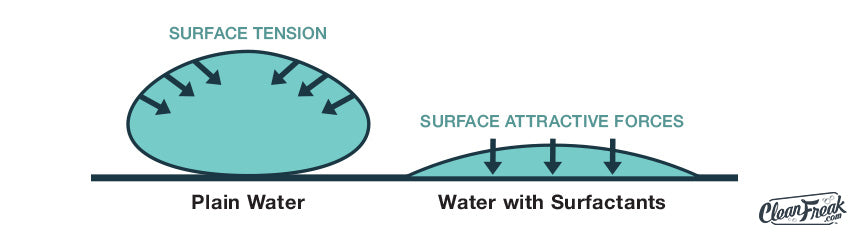
Cleaning detergents contain surfactants or “surface-active agents” that act to lower the surface tension of water. This allows the water to spread over your cleaning surface more quickly. As someone explained it, “Cleaning solutions make water wetter.” Sounds funny, but it’s a simple statement that condenses down the scientific principles.
Much of the everyday “dirt” that we encounter is oil based, and if there’s one thing we know, it’s that oil & water don’t mix. Oil-based stains will actually repel pure water, so water alone doesn’t cut it. The surfactants in the detergents we use can have positive and negative charges just like magnets. The end of the surfactant that is repelled by water is attracted to the soils. The opposite end that is attracted to water pulls the soil up off of the surface that we are cleaning. This push/pull effect (combined with hot water to dissolve the oil as well as agitation applied) helps to suspend the dirt and oil in water where it can be carried away.
There are other ingredients in cleaning solutions that can increase our cleaning efficiency. These work by altering pH levels or contributing to the stability of the solution and even preventing soils from re-depositing, but it all begins with the surfactants altering the chemical properties of the water. Let us boost your water’s cleaning power by adding the right chemical solution.


 Protect & Save with SuperFreak.
Protect & Save with SuperFreak.